In the framework of the ERC SOLCRIMET project SIM² KU Leuven researchers have found that replacement of water by ethylene glycol provides enhanced selectivity in the solvent extraction of transitions metals from rare earth elements. These findings have now been published in an Open Access paper in the Journal Separation and Purification Technology (Leuven, 2-4-2018)

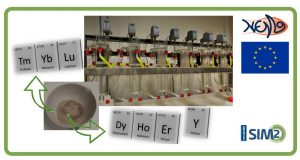
Graphical abstract demonstrating the principle of non-aqueous solvent extraction (SX)
Paper rationale
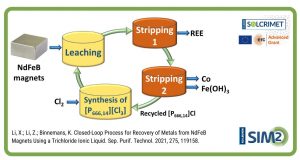
Conventionally, solvent extraction of metals consists of one organic phase containing extractants and one aqueous phase containing the metals to be separated. In fact the aqueous phase is not a must, as long as two immiscible phases can be formed, solvent extraction of metals can be performed.
In this work, the conventional aqueous phase was replaced by an ethylene glycol solution for the separation of transition metals from rare earth elements, which is relevant to the recycling of critical metals from secondary resources. The organic phase was Aliquat 336 (a commercial alkyl ammonium chloride extractants) dissolved in toluene. Compared with the extraction from the aqueous solutions, the non-aqueous solvent extraction from the ethylene glycol solution showed higher efficiency for the transition metal chlorides which is because that the complexes of transition metal chlorides are more readily formed in the ethylene glycol solution; while the rare earth chlorides were not extracted at all through they can be extracted from the aqueous solutions, the difference can be attributed to the weak salting-out effect of the ethylene glycol solution.
In principle, any metal ion that can form an anionic complex with chloride ions can be effectively separated from the rare earths by non-aqueous solvent extraction from the ethylene glycol solutions with Aliquat 336.
Full reference paper
Zheng Li, Xiaohua Li, Stijn Raiguel, Koen Binnemans, Separation of transition metals from rare earths by non-aqueous solvent extraction from ethylene glycol solutions using Aliquat 336, Separation and Purification Technology, 201, 318–326 (2018) – https://doi.org/10.1016/j.seppur.2018.03.022
Acknowledgements
The research leading to these results received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET).
Bio main author

Dr. Zheng Li (LIC, SIM² KU Leuven)
Dr. Zheng Li is a postdoctoral researcher working with Prof. Koen Binnemans in the group of LIC at SIM² KU Leuven. He obtained his PhD from the University of Melbourne in Australia in March 2016 and started to work on SOLCRIMET project at KU Leuven from May 2017.

 European Training Network for the Design and Recycling of Rare-Earth Permanent Magnet Motors and Generators in Hybrid and Full Electric Vehicles (DEMETER)
European Training Network for the Design and Recycling of Rare-Earth Permanent Magnet Motors and Generators in Hybrid and Full Electric Vehicles (DEMETER)