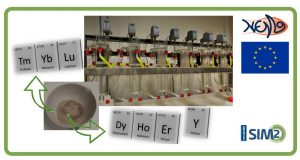
Non-aqueous solvent extraction is based on the distribution of metal ions between two immiscible organic phases, in contrast to conventional solvent extraction where the metal ions are distributed between an aqueous phase and an immiscible organic phase. Very few non-aqueous solvent extractions systems have been described in the literature, but it offers opportunities to develop new separation processes for metal ions. Kumar Batchu and Koen Binnemans developed a solvent extraction system where the more polar organic phase was ethylene glycol with dissolved rare-earth nitrate salts and lithium nitrate, while the less polar phase was a solution of the neutral extractant Cyanex 923 dissolved in n-dodecane. When compared to aqueous feed solutions, the light rare-earth elements (LREEs) are less efficiently extracted and the heavy rare-earth elements (HREEs) more efficiently extracted from an ethylene glycol feed solution, resulting into the easy separation of HREEs from LREEs. The separation factors between neighboring elements are higher for this non-aqueous solvent extraction process than for extraction from an aqueous feed solution.
Full reference: N.K. Batchu, T. Vander Hoogerstraete, D. Banerjee, K. Binnemans, Non-aqueous solvent extraction of rare-earth nitrates from ethylene glycol to n-dodecane by Cyanex 923, Separation and Purification Technology, 174, 544–553 (2017), doi: http://dx.doi.org/10.1016/j.seppur.2016.10.039

 European Training Network for the Design and Recycling of Rare-Earth Permanent Magnet Motors and Generators in Hybrid and Full Electric Vehicles (DEMETER)
European Training Network for the Design and Recycling of Rare-Earth Permanent Magnet Motors and Generators in Hybrid and Full Electric Vehicles (DEMETER)